INTRODUCTION: The antiphospholipid syndrome (APS), initially described nearly 4 decades ago, remains enigmatic with unclear pathophysiology. In addition to the lack of a mechanistic diagnostic test, APS exhibits considerable phenotypic heterogeneity, including the occurrence of “non-consensus” clinical manifestations (i.e. other than vascular thrombosis and pregnancy complications). Despite the evidence that phospholipid-binding factors are highly likely to be central to APS pathogenesis, the full complement of phospholipid-binding proteins has yet to be systemically assayed. Proteomic profiling by label-free quantitation (LFQ) may identify patterns of protein binding to phospholipid that are central to APS pathogenesis and thereby elucidate a basis for the heterogeneous clinical presentations of APS.
METHODS: C18 silica beads were coated with phospholipid by incubation with a suspension of 30% phosphatidylserine/70% phosphatidylcholine. Plasmas from 4 groups: 1) APS patients (n=11), 2) APL positive patients who lacked clinical manifestations (“APL”) (n=11), 3) non-APL patients with thrombosis (“nonAPL”)(n=11), and 4) normal healthy controls (“normal”) (n=6) were incubated with the phospholipid-coated beads, and eluates were processed by gel electrophoresis followed by tryptic digestion. The resulting peptides were analyzed by LC-MS/MS on a Thermo Orbitrap Fusion Lumos. Protein identification, false discovery rate (FDR) estimation, and LFQ were performed using Proteome Discoverer 2.2 (Thermo). LFQ values were normalized by overall peptide abundance, and relative quantitation was obtained. Groupwise comparisons were performed using PD 2.2 in order to identify differentially enriched proteins.
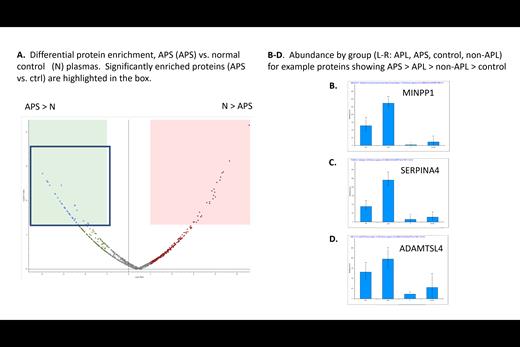
RESULTS: A total of 1,210 proteins were identified, with 875 proteins identified at high confidence (q<0.01). Based on gel electrophoresis, all samples had comparable amounts of total protein, and normalization of LFQ data on the basis of total proteins was therefore performed. Differentially expressed proteins between groups were identified using both fold-change and -log p value cutoffs (Figure 1a). Primary analysis focused on identifying proteins that were consistently preferentially bound from the APS plasmas compared to the normal controls.
Novel findings included the following proteins related to coagulation, immune response, and to lipid binding that bound preferentially from APS plasmas compared to normal plasmas (APS:control ratios shown in parentheses): coagulation factor VII (10.4), heparin cofactor 2 (9.6), CD14 (6.1), apolipoprotein L1 (6.0), and apolipoprotein L2 (5.5).
Additional proteins that preferentially bound from APS plasmas included OAF (13.7), BRSK1 (13.2), MINPP1 (12.5), fetuin B (10.5), SHBG (8.9), ADAMTS-like protein 4 (8.5), SERPIN A4 (7.6), alpha-2-HS glycoprotein (6.7) and inter-alpha-trypsin inhibitor heavy chains (H3, H4 and H1; 8.5, 6.6, 6.5 respectively).
Consistent with prior knowledge of APS, and validating this LFQ approach immunoglobulins and complement proteins were also among the preferentially bound proteins and included: Ig heavy chain 1-3 (15.6), complement C9 (13.3), complement C4-B, (7.1), complement component C8 beta chain (5.6), Ig kappa variable 1D-8 (5.6), and Ig lambda-like polypeptide 1 (3.9).
Interestingly, a number of identified proteins showed intermediate abundance (between APS and control) in the APL positive patients without clinical manifestations (“APL”) and APL negative patients with thrombosis (“nonAPL”) (see figure 1B-D for examples), suggesting a potential dose response effect.
CONCLUSIONS: 1) These results are a first demonstration of concept for the ability of LFQ proteomics to address the complexity of APS through analysis of phospholipid-bound proteins. 2) A number of phospholipid-binding proteins, many of which have not been previously implicated in APS pathogenesis, are preferentially present in APS patient plasma 3) Some plasmas from APL and non-APL patients also show increased binding of some of these proteins compared to the normal controls, suggesting that LFQ may identify markers for subgroups of patients within these 2 categories.
Disclosures
Master:Indigo Bioanalytical: Membership on an entity's Board of Directors or advisory committees; MEDAcorp: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal